Immunobiology and Pathophysiology of Hodgkin Lymphomas
As a medical student at the University of Groningen in the Netherlands, the author was intrigued to see a young man with Hodgkin Lymphoma, a lymphoid cancer that is characterized by large multinucleated tumor cells and a prominent inflammatory infiltrate. The author wanted to know how it was possible that a tumor could have less than 1% of tumor cells with such a huge majority of reactive cells and still be fatal in some patients.
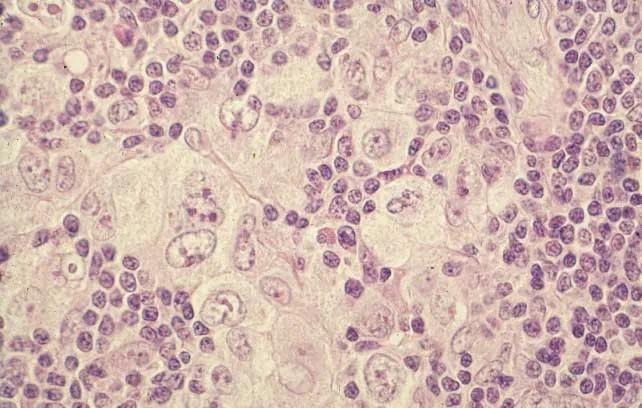
Microscopical slide showing a mixture of large multinucleated tumor cells and many reactive small lymphocytes with a few eosinophils, histiocytes and plasma cells.
The questions were about the origin of these tumor cells and the functional activity of the surrounding cells. It turned out that the nature of the tumor cells, termed Reed-Sternberg cells, was unknown and that the lymphocytes were thought to mount an immune response against the tumor cells. Were they actually mounting an effective immune reaction? This took place in 1974, during the last year in medical school, and the author decided to train as a pathologist and at the same time to pursue a PhD studying Hodgkin lymphoma. In the early seventies, immunology became a real science, the distinction between B-lymphocytes, the producers of antibodies and T-lymphocytes that interacted directly with virus infected cells or cancer cells had just been described. In the following years it became clear that there are different types of T-lymphocytes, some that help B-lymphocytes or other T-lymphocytes, some that suppress other lymphocytes and some that can directly kill virus infected cells or cancer cells. All of this is more relevant than ever when one is thinking about the immune response to COVID-19 infection and the effects of vaccination. For the questions about Hodgkin lymphoma, this knowledge on immunology meant that it became possible to identify different cell types in the tumor by using reagents that would recognize different types of lymphocytes. Hence, the author developed new immunostaining techniques that enabled the recognition of B-lymphocytes and T-lymphocytes cells in tissue sections. It became clear very soon that the neoplastic cells of Hodgkin lymphoma, the so-called Reed-Sternberg cells, giant cells that are unlike any other cells in the body, could not be identified by these methods as being either T- or B-lymphocytes. However, the big majority of the cells surrounding the Reed-Sternberg cells were T-lymphocytes, although one could not yet resolve whether these were helper cells or suppressor cells or killer cells. The author also decided to work for a while in the University of Kiel in Germany because Professor Karl Lennert was the world expert on lymphomas then, and to learn from him. Together a series of papers were produced describing different types of Hodgkin lymphoma and their cellular composition. The author defended his thesis on the immunopathology of Hodgkin lymphoma in 1979 and next went on to do a postdoc at Harvard Medical School. The Nobel Prize winning invention of the hybridoma technology by Köhler and Milstein enabled Schlossman and Reinherz at Harvard Medical School to develop monoclonal antibodies that could recognize specific T-lymphocyte subsets. The next step was to develop the methodology to apply these on tissue sections so we could recognize T-lymphocyte subtypes types in situ. It turned out that the T-lymphocytes immediately surrounding the Reed-Sternberg cells were helper T-lymphocytes, suggesting that these cells do not attack or suppress the tumor cells, but rather helped them survive.
This demonstrates the frequent dependence on new technology to take the questions a step further. It is mentioned above that it was not possible to identify what type of cells the Reed-Sternberg cells were. However, when the author was invited to give a lecture at the NIH in 1980, one scientist in the audience informed about the possibility to use molecular biological methods to detect rearrangements of the immunoglobulin genes or the T-cell receptor genes which would allow the identification of B- or T-cells, but also the demonstration whether these cells were clonal, indicating a tumor, or not. Back in Groningen, the author set up his own molecular lab in addition to the hybridoma lab that was already established. In the hybridoma lab, a series of new monoclonal antibodies was produced and commercialized which provided the money to build the molecular lab. And then, it could be demonstrated that Reed-Sternberg cells had monoclonal immunoglobulin rearrangements, but that these rearrangements were defective and did not result in the production of messenger RNA and antibodies. This answered the question of the nature of Reed-Sternberg cells. They were neoplastic, crippled B-lymphocytes and the cells surrounding them were T-lymphocytes that helped these crippled neoplastic cells survive.
The next question was what was causing this peculiar transformation of B-lymphocytes to Reed-Sternberg cells. There had been suspicions that the Epstein-Barr virus might be involved, the same virus that causes infectious mononucleosis but also Burkitt lymphoma and several other neoplasms. Of relevance was that infectious mononucleosis contains similar multinucleated giant cells of B cell origin as Hodgkin lymphoma. It was possible then to demonstrate the presence of EBNA-1 (Epstein-Barr virus Nuclear Antigen-1) in the nuclei of Reed-Sternberg cells with an immunohistological method.
So how then could it be that Reed-Sternberg cells were expressing EB virus antigens but were escaping an effective immune response? The recognition of viral antigens is dependent on the proper presentation of the antigen by the human leucocyte antigen (HLA) system, so the decision was to study whether there were any specific changes in the HLA system of EB virus positive Hodgkin lymphoma cases and it turned out indeed that these cases had infrequent expression of HLA A2 which does present an EBV antigens effectively and a frequent expression of HLA A1 which does not present EBV antigens. This partly explains why there is no effective reaction of T cells killing the tumor cells.
In later studies, a new technology termed SAGE (Serial Analysis of Gene Expression) to find genes that were highly expressed in Reed-Sternberg cells was employed. This included DNA coding for proteins such as the chemokine TARC that is specifically attracting T-helper cells, providing an explanation for the prominent presence of these cells in Hodgkin lesions. Serum levels of TARC are currently used as a marker for the activity of Hodgkin lymphoma. The research team was also the first to find a high expression of MicroRNA-155 that promotes lymphomagenesis.
Much of the research has been focused on the various mechanisms that Reed-Sternberg cells use to escape an effective immune response and to induce a response that creates a tolerant environment for the tumor cells and to devise methods that will break this tolerance. Interestingly, this provided the tools that could be used to study the other side of the medal. In organ transplantation, the goal is to induce tolerance and several of the mechanisms that Reed-Sternberg cells are using to induce tolerance can be used to induce tolerance in transplantation. However, this would be another story.
In conclusion, this research was inspired by questions. Answers to these questions led to new questions. Progress was frequently dependent on the development of new technologies, such as hybridoma technology, gene rearrangement analysis, gene expression analysis etc. Successful research requires focus, a willingness to learn from the best, and collaboration. Many thanks are owed to the professors and supervisors in Groningen, Kiel and Harvard and to a long line of PhD students that have been involved in these studies over the years. "
Professor Sibrandes Poppema
Chancellery Office
Email: @email